
General features: It is a perennial flowering vine with the botanical name Piper chaba (PC), commonly known in English as Piper chilli or Indian long pepper. The plant belongs to the Piperaceae family of the Piper genus. The family possesses nearly 2000 dominant species. The appearance of the leaves and plant is identical to the Betel leaf plant (Paan – Bengali)[https://my-herb.org/2023/09/10/betel-leaf-paan-%e0%a6%aa%e0%a6%be%e0%a6%a8-bengali-hindi/]. PC is a tropical plant. It originates from Southeast Asia, mainly India, Sri Lanka, Indonesia, Malaysia, Thailand, Bangladesh, and Singapore. The plant is a highly favored spice for cooking various kinds of soups (Jhole in Bengali) in West Bengal and Bangladesh, particularly in and around the Khulna district, colloquially named “Choi.” In ancient Ayurveda, it is called Pippali and is thus considered to be an Ayurvedic plant widely famed in Indian traditional medicine for its beneficial role in curing numerous ailments, including indigestion, respiratory problems, and several health issues [1]. Besides the medicinal role, PC is normally used for culinary purposes, mostly in India and Bangladesh, especially in West Bengal, Tripura, and the Khulna district of Bangladesh. Likewise, in West Bengal, it also grows in Madhya Pradesh of India. The plant is a creeper by nature, so it can spread on the ground or wrap around a large tree to rise high, about 20 ft. The leaves, stems, fruits/flowers, and roots are used in a variety of Bengali dishes. The leaves are oblate or oval-shaped, 2 – 3 inches long. The fruits are almost identical to long pepper, 2 – 3 inches long, which is a different type of long pepper or hot chilli, but with the same name, Pippali. It turns red when ripe, otherwise whitish in appearance if young. When dried, it turns dark brown or black. It provides a warm and hot sensation when used in cooking, offering a strong spicy taste very similar to Black pepper, due to the presence of piperine and its derivatives. In Asian villages, the plant is frequently utilized to heal common ailments.
Folk medicinal uses: It is mentioned in the Ayurveda (Gajayurveda, treatments for elephants. ‘Gaja’ means elephant in Sanskrit) that Piper chaba is one of the five items of Panakola, an ancient Ayurvedic concoctionused in the treatment of big animals, particularly elephants or horses. Panakola contains five ingredients: Piper longum, its roots, Piper chaba, Piper cubeba, and dried ginger [https://www.wisdomlib.org/definition/piper-chaba]. Besides animals, it has also been introduced to treat humans. Thus, from very ancient times, the Piperaceae family is highly acclaimed for its versatile medicinal role [2]. The traditional functions rely on its ability to reduce pain, inflammation, and various infections. The unripe fruit or flower has a hot, spicy, aromatic character identical to Black pepper. It acts as an expectorant and exhibits diuretic properties, even removes dizziness, headaches, hysteria, paralysis, and memory loss, also acts as a remedy in case of catarrh and gleet [3]. The ethanolic extract shows a cholesterol and lipid-lowering effect. The fruit extract indicates anxiolytic, antifungal, antitussive, erythropoietic, and appetizing activities. In Thailand, the plant is often used to treat asthma, indigestion, bronchitis, and arthritis. In Southeast Asian villages, it also finds application to preserve food [3]. In the Indian subcontinent, different parts of the PC are exploited to treat various ailments. The aerial part exhibits anti-diarrheal, antibacterial, anti-hypertensive, carminative, diuretic, stimulant, expectorant, analgesic, and smooth muscle relaxant properties [4]. The stem bark extract provides strong anti-inflammatory actions, tested on rats [5]. Additionally, the extract also offers other beneficial effects, acting as antihyperlipidemic, anti-androgenic, immunoregulatory, and anti-depressant [6]. Owing to its strong analgesic property, it is even used to reduce post-delivery or rheumatic pain [4].
Culinary uses: PC is a trendy spice in Southeast Asian countries, popularly known as “Choi.” Its pungent, hot, and spicy taste closely resembles Horseradish and black pepper, although somewhat floral. It is a well-liked ingredient, therefore used in many Thai sauces and pastes for preparing soups. Both fresh and dried forms are utilized to cook any Thai dish, particularly fish [7]. The stems, roots, leaves, and bark/skin are typically used in the cooking of meat or fish. The leaves are even used to wrap food to provide it with a spicy flavor [6]. The mashed stems and roots are also applied to season meats and vegetables. In Bengal, the chaba stems are typically used in curries, whereas the fruits are added to lentil soups or vegetable stews to impart a spicy aroma.
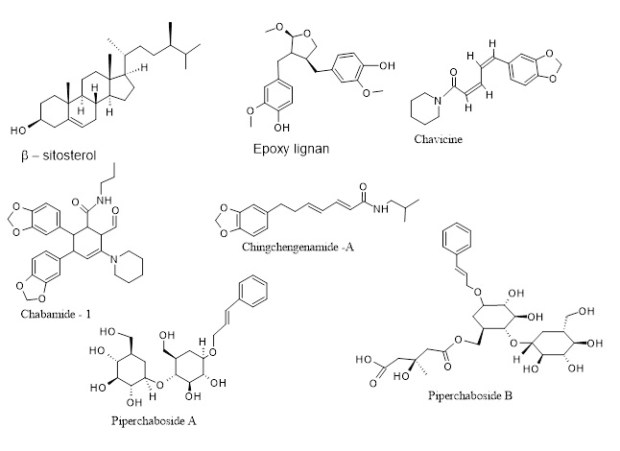
Phyto-chemicals and medicinal roles: Loads of phytochemicals of versatile natures have been identified in different parts of the plant body. From the stem bark, a few isoflavans are isolated, and in addition, there are kusunokinin and an alkaloid, pellitorine. β-caryophyllene and caryophyllene oxide are identified in fruit oil. Whereas piperine, pipernonaline, guineensine, isobutylamide, and some others are from the flowering plant. A few new alkaloids, some of which are dimeric, have been identified from the roots. They are chabamide and its derivatives, like chabamide F, chabamide G, with pyrrolidone rings [7,8]. By far the most extensively studied compounds in all piper species are Piperlongumine and Piperlartine, the alkaloid amides having strong anticancer properties [9]. It has been noted that the essential oil shows several pharmacological actions exhibiting antiproliferative and antimicrobial properties [9]. Natural sesquiterpenes, Caryophyllene and its derivatives, have a preventive role in neurodegenerative and neuroinflammatory diseases like Alzheimer’s, owing to their actions on the CB2 receptor. It also triggers the peroxisome proliferator-activated receptor-γ (PPARγ) pathway [10]. Piperchabamides have a hepatoprotective role, inhibiting the death of toxin-induced hepatocytes. They also possess antitumor actions [11]. The well-known hot pepper ingredient, Piperine, exerts digestive support along with anti-inflammatory, antioxidant, and anti-tumor effects [12]. It also produces antidepressant activity by inhibiting monoamine oxidases (MAO–A and MAO–B), thereby increasing the levels of monoamines, inducing antidepressant actions which have already been established experimentally in laboratory mouse models [13]. The compound also upregulates hippocampal progenitor cell proliferation, causing neurogenesis in mice under chronic stress, helping with learning behavior and memory [14]. Chabamide F and G show great potential in breast cancer treatment. They also have anthelmintic, antifungal, antibacterial, and herbicidal effects [15]. Kusunokinin is a unique lignan that has a strong anticancer role, particularly the (-) isomer [16]. Guineensine inhibits the uptake of endocannabinoids. Additionally, it has larvicidal, anti-inflammatory, and cholinesterase inhibitory properties [17]. Sesamin is another lignan, and it has versatile roles while acting as an antioxidant, anti-inflammatory, and anti-cancer agent. It also regulates blood pressure, improves liver function, and subsequently reduces lipid levels in circulation [18]. On the other hand, β-sitosterol, a widely available phytosterol, is known to have a significant role in controlling benign prostatic hyperplasia. It further reduces the risk of cardiac problems and lowers the absorption of cholesterol in the intestines. Additionally, it has a strong immunomodulatory property and anti-inflammatory and anticancer effects [19]. There are other lignans in PC that have multiple roles in health. They have a resemblance to the steroid moiety and, by acting as phyto-estrogen, enabling them to reduce heart diseases, osteoporosis, menopausal symptoms, and breast cancers [20]. Chavicine, an alkaloid and isomer of piperine, is a pungent, tasteless compound. Like piperine, it exerts potent immunomodulatory, antioxidant behavior. In addition, it also shows anti-asthmatic, anti-inflammatory, anti-ulcer, and anti-amebic effects [21]. Several phytochemicals have been identified in the PC (Pipla) cultivated in West Bengal, India; Trans-piperitone oxide, Piperidine, Piperonylic acid, Piperyline, (-)-piperitone, cis-piperitol, piperitenone oxide, etc [22]. Chabamide-1, a newly isolated alkaloid from PC, shows anti-inflammatory, anti-diarrheal, and anti-microbial activities; in addition, it demonstrates antitumor actions by exerting cell cycle arrest, apoptosis, and autophagy in cancer cells [23]. The other alkaloid, Chingchengenamide A, also produces antidiarrheal, antimicrobial, and pain-relieving effects like Chabamide-1 [24]. Pellitorine, one of the major ingredients of PC, is a potential anti-cancer compound showing strong cytotoxic activity when tested on HL-60 (human leukemia) and MCF-7 (human breast cancer) cell lines [25]. It also has antithrombotic, anti-inflammatory, and numbing roles. The numbing action is due to its inhibitory or antagonistic effect on the TRPV1 receptor; therefore acts as a medicine for relieving pain [26]. Pipernoline shows potent antidiabetic activity, stabilizing blood sugar. It also shows a large potential to improve nutrient absorption, anti-cancer, and brain boosting functions, improving memory, and lowering amyloid plaque. It even exhibits cardioprotective activity [27]. The other alkaloid, Pipernoline, has very similar roles to piperine and its derivatives, which include antioxidant, anti-inflammatory, anti-diabetic, and brain-boosting effects [27]. The isoflavones are known to exert beneficial roles while acting as phytoestrogens, interacting with estrogen receptors and simultaneously reducing inflammation. They also exert beneficial effects on bone health, cardiac health, and the prevention of several cancers [28]. The piperchabaosides A and B are glycosides composed of glycone and aglycone. They also show diverse therapeutic actions, exerting anti-inflammatory, anticancer, analgesic, antidiabetic, and antimicrobial effects [29].

Pharmacological effects: Owing to its large reserve of numerous phytochemicals, PC exhibits a huge number of pharmacological activities.
Cytotoxicity and anticancer effects – The petether-chloroform extract of roots shows cytotoxicity against Artemia salina (Brine shrimp), IC50 = 0.76 – 1.53 µg/ml. The chloroform extract of the fruits displays toxicity against Entamoeba histolytica (intestinal amoebiasis), IC50 = 71.4 µg/ml [29]. Cytotoxic behavior of PC is also noticed in the case of many cancer/tumor cells. The ethanol extract of fruits exerts significant actions on several cancer cell lines like CL-6 (IC50 = 50.62 ± 3.10 µg/ml), HepG2 (IC50 = 72.25 ± 1.15µg/ml), Hep 2 (IC50 = 12.42 ± 0.99 µg/ml), and HRE (119.14 ± 9.94 µg/ml) [30]. It has been noticed that Chabamide-1, being the most effective component, inhibits the growth of K562/ADR cells in a dose and time-dependent manner. It suppresses cell proliferation by arresting them at the G0/G1 phase. The event is associated with an increase in p21 and a decline in cyclin D1 and CDK2/4/6 expression. The compound also changes the mitochondrial membrane potential and simultaneously enhances expression of apoptosis-related proteins like Bcl-2, Caspase-3 & 9, PARP-1, and p-Akt. It even regulates the c-Jun N-terminal kinase (JNK), ERK1/2, and p38. It also induces autophagy [31,32].
Hepatoprotective effect – The aqueous-acetone fruit extract, and also piperine, dose-dependently provide hepatoprotective effects against D-galactosamine/lipopolysaccharide-induced liver injury in mice [33]. It is noticed that piperine reduces the sensitivity of TNF-α toward hepatocytes in a dosedependent manner. In that way, piperine exerts a considerable hepatoprotective effect in laboratory animals [34]. Further, piperine stimulates LDL receptor gene expression and enhances LDL uptake [35].
Gastroprotective effect – The compounds piperine, pipernonaline, dehydropipernonaline, retrofractamide B, N-isobutyl-(2E,4E)-octadecadienamide, and N-isobutyl-(2E,4E,14Z)-eicosatrienamide at a 25 mg/Kg dose peritoneally exert significant gastroprotective effects in ethanol and indomethacin-induced lesions in rats [36].

Antidiabetic effect – Two isomers, cis– and trans-piperlartine of PC, are observed to inhibit recombinant human aldose reductase(ALR2) with an IC50 of ~ 160 µM, demonstrating its antidiabetic actions [37]. ALR2 is a rate-limiting enzyme of the polyol pathway in glucose metabolism, reducing excess glucose by catalyzing NADPH-dependent conversion to sorbitol. In diabetes, ALR2 activity increases, converting excess glucose into sorbitol in insulin-independent tissues, such as the kidneys, nerves, retinas, and lenses. The high accumulation of sorbitol plays a strong adversarial role, enhancing diabetic complications like neuropathy, retinopathy, and cataract. So, this inhibitory effect indicates that ALR2 could be a potential target to control diabetic complications, and inhibiting it could be beneficial to diabetic patients [37]. Further, the role of piperine or the extract of PC in STZ-induced diabetic mice indicates that it is able to lower the fasting blood glucose level to a considerable extent. It also increases the level of superoxide dismutase while decreasing the malondialdehyde. In addition, the compound significantly reduces the apoptotic rate of pancreatic β-cells and expression ratio of Bax/Bcl-2 while upregulating the expression of PI3K and AKT in the pancreas. In a way, it has a huge beneficial role in controlling diabetes [38].
Adipogenesis effect – Studies have shown that several compounds isolated from PC, such as piperlonguminine and refractomide A, B, and C, can promote adipogenesis in the preadipocyte mouse fibroblast cell line, 3T3-L1 cells. Among those, refractomide A is noted to be more active, while significantly increasing adiponectin release and subsequent uptake of 2-deoxyglucose within cells. The compound, refractomide A, also enhances the mRNA expression level of adiponectin, PPARγ2, and glucose transporter GLUT4, as well as insulin receptor substrate1(IRS-1), lowering the risk of diabetes [39]. Almost similar effects are also observed for several other phyto-components of PC [40,41]. Below are the components involved in adipogenesis.

Anti-bacterial, anti-fungal, anti-leishmanial, and anti-malarial effects – Numerous compounds of PC have revealed their multiple pharmacological roles. For example, chabamide from the stem extract exhibits potent antibacterial activity against Mycobacterium tuberculosis (H37Ra), with a minimum inhibitory concentration (MIC) of 12.5 µg/mL [42]. Furthermore, Bornyl piperate and piperlonguminine from the root extract produce strong inhibition against Bacillus subtilis, Bacillus megaterium, Staphylococcus aureus, Staphylococcus β-haemolytcus, Escherichia coli, Salmonella typhi, Shigella dysenteriae, Shigella shiga, and Shigella boydii [43].

It is noticed that the fruit extract by methanol can inhibit nine out of ten human and animal pathogens [44]. Studies have further shown that the compounds bornyl piperate and piperlonguminine possess a significant antifungal activity against Aspergillus fumigatus, Aspergillus niger, Aspergillus flavus, and Candida albicans [44]. Uniquely, these two compounds also exert strong preventive activity against Leishmania donovani, with IC50 values of 16 and 30 µg/ml [44]. Regarding the anti-malarial effect, studies conducted using Thai traditional medicine show that the ethanol extract of PC is exceedingly active against chloroquine-resistant (K1) and chloroquine-sensitive (3D7) P falciparum with IC50 values of 5.3 and 4.1 µg/ml, respectively [45]. Recent studies have further established that piperine, the major component of PC, has strong activity against both kinds of malarial strains [46]. On the other hand, it has also been noticed that the methylene chloride extract of fruit is highly active against the parasite, Schistosome cercariae [47].
Anti-inflammatory effect – The ethanol extract of PC stem inhibits ethyl phenyl propiolate-induced ear edema and carrageenan-induced hind paw edema in rats. It also reduces transudative and granuloma weights, as well as body weight gain and thymus weight in the case of the chronic inflammatory model created by cotton pellet-induced granuloma formation in rats. The extract also shows analgesic actions in the formalin test and antipyretic activities in yeast-induced hyperthermia in rats [48]. The compounds bornyl piperate, piperlonguminine are identified to be the most effective components. The anti-inflammatory actions are mediated via the stabilization of the cell surface membrane [49]. Further, chabamide also produces an anti-inflammatory effect via the activation of Nrf2/heme-oxygenase-1 pathway, which is noticed in the murine macrophage cell line, RAW264.7 [50].
Antioxidant effect – Antioxidants halt the chain reaction that occurs during oxidation in the context of oxidative stress, enabling the removal of free radical intermediates and thereby inhibiting the oxidation process. The effect extends to prevent cardiac problems, cancers, Alzheimer’s disorders, rheumatoid arthritis, and cataracts [51]. In PC, the isoflavones, alkaloids, and many others act as strong antioxidant compounds. The DPPH free radical scavenging activity of isolated pure piperine is IC50 ~ 5.14µg/ml [6].
Hypolipidemic effect – Using albino rabbits weighing 1- 1.5 Kg, the administration of ethanolic extract of PC, 160 mg/Kg, shows significant lipid-lowering activity when measured LDL, cholesterol in the circulating blood, subsequently enhancing the HDL level [52]. In alloxan-induced diabetic rats, intraperitoneal injection of the ethanolic plant extract shows hypoglycemic effect along with the lowering of triglyceride, total cholesterol, LDL, and VLDL with simultaneous increase of HDL levels [53]. Further study has established that a new piperine derivative, GB-N from PC, when orally administered in hyperlipidemic rats (2.5 – 10 mg/Kg), significantly lowers the levels of serum TG, enhances HDL-C, and upregulates hepatic HMG-CoA reductase, LDL-receptor mRNA expression [54].

Miscellaneous actions – Diuretic, Antihypertensive, anti-ulcer, Analgesic, Anti-diarrheal, Anti-depressant, and Anti-gastrointestinal motility [29].
Conclusion: Due to its vast reservoir of versatile phytochemicals, Piper chaba shows immense potential for therapeutic uses. Research further indicates its huge pharmacological activities, while showing the least toxicity [55]. In that perspective, this herb can be used as a complementary medicine for treating numerous diseases.
Bibliography
- Rameshkumar K B, Aravind A P, Mathew P J. J Herbs, Spices Med Plants 2011, 17(4), 351 – 360. https://doi.org/10.1080%2F10496475.2011.632116
- Salehi B, Zakaria Z A, Gyawali R et al. Molecules 2019, 24, 1364. http://dx.doi.org/10.3390/molecules24071364
- https://www.phytomednepal.com/plant/piper-chaba-hunter#:~:text=Ethnobotanical%20Uses,in%20case%20of%20gleet%2C%20catarrh.
- Yusuf M, Chowdhuri J U, Begum D J. “Medicinal Plants of Bangladesh” – Bangladesh Council of Scientific and Industrial Research; 1994, p 192.
- Rahman M T U, Shilpi J A, Ahmed M et al. J Ethnopharmacol 2005, 99, 203 – 209.
- Haque M E, Roy A N, Rani M. Int J Sci Eng Res, 2018, 9(3), 937 – 943.
- https://www.goya.in/blog/choi-jhal-a-forgotten-spice-from-khulna
- Shandhi S P, Richi F T, Alam S et al. Food Aci Nutri 2024, 12, 10680 – 10698. https://doi.org/10.1002/fsn3.4585
- Bezerra D P, Ferreira P M P, Machado C M L et al. Planta Med 2015, 81, 1730 – 1739.
- Cheng Y, Dong Z, Liu S. Pharmacol 2014, 94(1), 1 – 12.doi: 10.1159/000362689.
- Jeon H J, Kim K, Kim Y D et al. App Biol Chem 2019, 62, 63. https://doi.org/10.1186/s13765-019-0471-z
- Bahadi D, Dadashzadeh S, Shahsavari Z et al. Int J Pharm 2022, 624, 121990.
- Lee S A, Hong S S, Han X H et al. Chem Pharm Bull, 2005, 53(7), 832 – 835. https://doi.org/10.1248/cpb.53.832
- Yau S, Li A, So K F. Neural Plast 2015, Art ID 717958. http://dx.doi.org/10.1155/2015/717958
- Rao V R, Ganji S, Sarma V U M et al. Tetrahed Lett 2009, 50(23), 2774 – 2777.
- Rattanaburee T, Thongapanchang T, Wongma K et al. Biomed Pharmacotherap, 2019, 117, 109115. https://doi.org/10.1016/j.biopha.2019.109115
- Nicolussi S, Viveros-Paredes J, Gachet M S et al. Phramcol Res, 2014, 80, 52 – 65.doi: 10.1016/j.phrs.2013.12.010
- Majadalawieh A F, Nasarallah G. Eu J Pharmacol, 2017, 815, 15, 512 – 521.
- Pizzorono J E, Murray M T, Joiner-Bey H. “The Clinician’s Handbook of Natural Medicine” – 3rd Ed, 2016, 137 – 146. https://doi.org/10.1016/B978-0-7020-5514-0.00021-X
- Rodriguez-Garcia C, sanchez-Quesada C, Toledo E et al. Molecules 2019, 24(5), 917. https://doi.org/10.3390/molecules24050917
- https://pubchem.ncbi.nlm.nih.gov/compound/Chavicine
- https://neist.res.in/osadhi/detail.php?name=Piper+chaba#:~:text=Scientific%20Name%3APiper%20chaba,chaba%2C%20Piper%20officinarum%2Cetc.
- Hu K, Yang M, Xu Y Y et al. Chinese Herb Med 2016, 8(1), 30 – 38. https://doi.org/10.1016/S1674-6384(16)60005-9
- Parvin S, Richi F T, Alam S et al. Food Sci Nutri, 2024, 12(12), 10680 – 10698. http://dx.doi.org/10.1002/fsn3.4585
- Ee G C L, Lim C M, Rahman M et al. Molecules 2010, 15, 2398 – 2404. doi:10.3390/molecules15042398 .
- Olah Z, Redei D, Pecze L et al. Phytomed 2017, 34 (15), 44 – 49.
- Haq I U, Imran M, Nadeem M et al. Phytotherap Res 2021, 35(2), 680 – 700. doi: 10.1002/ptr.6855.
- Gomez-Zorita S, Gonzalez-Arceo M, Fernandez-Quintela A et al. Nutrients 2020, 12(12), 3853. https://doi.org/10.3390/nu12123853
- Islam M T, Hasan J, Snigdha H M S H et al. J Ethnopharmacol 2020, 257, 112853. https://doi.org/10.1016/j.jep.2020.112853
- Mahavorasirikul W, Viyanant V, Chaiiaroenkul W et al. BMC Compl Alternative Med 2010, 10, 55. https://doi.org/10.1186/1472-6882-10-55
- Ren J, Xu Y, Huang Q et al. Anti Canc Drugs 2015,26(5), 498 – 507.
- Hu K, Yang M, Xu Y, et al. 2016, Chinese Herb Med 8(1), 30 – 38. https://doi.org/10.1016/S1674-6384(16)60005-9
- Matsuda H, Nalamura S, Yoshikawa M. Biol Pharm Bull, 2014, 37(6), 884 – 891. https://doi.org/10.1248/bpb.b14-00037.
- Morinkawa T. Yakugaku Zasshi 2010, 130(6), 785 – 791.
- Ociai A, Miyata S, Shimizu M et al. PloS One,10(10), e0139799.
- Morikawa T, Matsuda H, Yamaguchi I et al. Planta Med, 2004, 70, 152 – 159. https://doi.org/10.1055/s-2004-815493
- Rao V R, Muthenna P, Shankaraiah G et al. Eu J Mef Chem, 2012, 57, 344 – 361.
- He Q, Xu J Y, Gu J et al. J Funct Foods 2022, 88,104090.
- Zhang H, Matsuda H, Nakamura S et al. Bioorg Med Chem Lett 2008, 18(11), 3272 – 3277. https://doi.org/10.1016/j.bmcl.2008.04.052
- Mourad A A, Nakamura S, Ueno T et al. Bioorg Med Chem Lett 2013, 23(17), 4813 – 4816.
- Yamaguchi I, Matsuda H, Zhang H et al. J Nat Med 2014, 68(1), 74 – 82. https://doi.org/10.1007/s11418-013-0770-3.
- Rukachaisirikul T, Prabpai S, Champung O Suksamaran A. Planta Med 2002, 68, 853 – 855. https://doi.org/10.1055/s-2002-34410.
- Naz T, Mosaddik A, Rahman M et al. Nat Prod Res, 2012, 26(11), 979 – 986. https://doi.org/10.1080/14786419.2010.535166.
- Panphut W, Budsabun T, Sangsuriya P. Internet J Microbiol, 2020, 5638961. https://doi.org/10.1155/2020/5638961.
- Thiengsusuk A, Chaijaroenkul W, Na-Bangchang K. Parasitol Res 2013, 112(4), 1475 – 1481. https://doi.org/10.1007/s00436-013-3294-6
- Thiengsusuk A, Muhamad P, Chaijaroenkul W, Na-Bangchang. J Trop Med 2018, 9486905. https://doi.org/10.1155/2018/
- Atianasuppat K, Wongkham W, Meepowpan P et al. J Ethnopharmacol 2009, 123(3), 475 – 482.
- Seewabon S, Arunporn I, Nusiri L. ISRN Pharmacol 2012, Article ID480265. doi:10.5402/2012/480265
- Yesmin S, Paul A, Naz T et al. Clin Phytosci 2020, 6, 59. https://doi.org/10.1186/s40816-020-00207-7
- Ngo Q M, Tran P T, Tran M H et al. Phytotherap Res 2017, 31(4), 663 – 670. https://doi.org/10.1002/ptr.5780.
- Sies H. “Oxidative stress: oxidants and antioxidants”, Experimental Physiology. 1997, Vol 82, 291 – 295.
- Sarafraz S, Najam R, Azhar I, Sarwar G. Forensic Biomechanics 2016,7(2), 128.
- Islam M M, Bari M W, Afroza D et al. World J Pharm Res 8(10), 203 – 215.
- Bao L, Bai S, Borijihan G.Pharm Biol 2012, 50(8), 962 – 967.DOI: 10.3109/13880209.2012.654395
- Jaijoy K, Vannasiri S, Piyabhan P et al. Int J Appl Res 2010, 3(4), 29 – 35.
