US Pharm. 2013;38(1):HS1-HS5.
ABSTRACT: Neurologic complications of ischemic
stroke remain the leading cause of disability in the United States.
Achieving cerebral reperfusion within 90 minutes of a stroke event
remains the optimal opportunity to prevent neurologic deterioration and
provide amelioration of most neurologic complications. Studies also
support extending the reperfusion window to 4.5 hours. Rehabilitation
remains the cornerstone to improve stroke sequelae outcomes, and
pharmacologic agents are useful in the management of neuromuscular,
cognitive, and mood disorder sequelae of stroke. Future therapies and
rehabilitation techniques include agents and techniques that will impact
neurodegeneration, achieve neuroprotection to decrease stroke infarct
size, and prevent or ameliorate the development of the neurologic
consequences of stroke.
Stroke is a focal cerebral insult that often results in
neurologic and medical sequelae with long-term implications for quality
of life, morbidity, and mortality. Stroke remains the third leading
cause of cardiovascular-related death in the United States. Ischemic
stroke caused by embolic events linked to athero-sclerosis represents
the majority of strokes (85%), and hemorrhagic stroke accounts for the
rest. Cardioembolic stroke makes up 30% of all ischemic strokes, while
small and large vessel occlusive and atherosclerotic diseases account
for 25% each.1
During ischemic stroke, the occluded vessel is often a
major cerebral artery. The injured area in the brain consists of a
central core region with ischemic lesions and the surrounding penumbra
areas. The cerebral tissues in the core are starved of oxygen due to
decreased blood flow and perfusion. Oxygen starvation of the cerebral
cells causes irreversible damage and cell death due to necrosis,
although some cells do die from apoptosis, a programmed cell death.2
Cells in the penumbra are often starved of oxygen, but many still
maintain membrane integrity, avoiding necrotization and death. The
severity and duration of the ischemia affecting cells in the penumbra
region determine whether the cell will survive or die.3
Timely reperfusion using therapeutic, interventional, or spontaneous
recovery represent the best way to salvage the cerebral penumbra region
and prevent cell death. It is worth noting that reperfusion and
restoration of blood flow to the cerebral penumbra in itself can cause
injury due to a surge in the generation of reactive oxygen species such
as proinflammatory neutrophils, which infiltrate and exacerbate ischemic
injury in the penumbra tissues.4
Hemorrhagic stroke results from intracerebral hemorrhage
in the subarachnoid space. Causes include brain aneurysm or
atrioventricular malformations in the dural space, bleeding from a
tumor, hematologic disorders, vasculitis, and drug abuse. The mortality
rate for hemorrhagic stroke is very high, and survivors can have minimal
to very severe deficits. The total cost of treating stroke was $34.3
billion in 2008.5 Fifty percent of the amount was due to loss
in productivity from stroke-induced disability including hemiparesis,
inability to walk, aphasia, mood disorders, and institutionalization.6 Stroke is the leading cause of long-term nonambulatory and total dependence disability in the U.S.5
NEUROLOGIC COMPLICATIONS
Neurologic Deterioration
Neurologic deterioration is a usual feature of stroke,
affecting half of all patients with manifested complications within 24
hours of the stroke episode.7 Acutely, these include brain
swelling; ischemic progression with recruitment and damage to
surrounding cerebral tissues; cerebral hemorrhage from accumulation of
small petechial hemorrhages; and hematomas, seizure, and death.7
Several factors can facilitate the neurologic deterioration; chief
among these is delay in initiating reperfusion therapy or failure of the
therapy to adequately reestablish reperfusion in the affected areas,
and/or the occurrence of reocclusion.8
Neuromuscular Dysfunction
Neuromuscular dysfunction affects many stroke patients,
causing disabilities such as apraxia, pain syndromes, limb spasticity,
and incontinence. Apraxia is a disorder of skilled movement caused by
weakness, akinesia, abnormal tone or posture, tremor or chorea,
intellectual deterioration, poor comprehension, or uncooperativeness.
Apraxia can affect the mouth, face, and eyelids.9 Pain
syndromes are musculoskeletal pain in stroke patients caused by poor
motor control, originating from the interaction of both neurologic and
musculoskeletal sequelae such as improper limb and gait biomechanics.
The pain can affect the shoulders, hips, muscles, and other parts of the
body. Hemiparetic shoulder pain (HSP) is a disabling, severe pain often
occurring on the side affected by hemiplegia or paralysis.10
HSP often is accompanied by limitation in range of motion in the
shoulder. Central poststroke pain (CPSP) is difficult to treat. This
pain is described as heightened distress in response to unpleasant
stimuli such as a pinprick. Spasticity of the limb is a stroke sequela
and a motor neuron disease that causes increased resistance to muscle
stretching capacity. Often the affected limb is initially in a limp
state before becoming spastic.9
Incontinence of the bladder or bowel impacts quality of
life for stroke patients and is frequently a significant indicator of
poor outcomes, disability, and the need for institutionalization.11
Factors that contribute to poststroke incontinence include direct
damage and disruption to the micturition centers in the brain, causing
bladder hyperreflexia and urgency. Normal bladder function may be intact
poststroke, but development of deficits impairing mobility and
cognition occurs in patients who cannot use the commode by themselves.
This is a common occurrence in those older than 75 years with comorbid
dysphagia and vision problems.9 Bowel and/or bladder
incontinence in a stroke patient may indicate negative prognosis when it
occurs long after the stroke event.12
Cognitive Impairment
Stroke negatively impacts patients’ cognitive abilities,
from memory loss to dysfunction in reasoning, speech, and
problem-solving skills. Cognitive dysfunction may manifest as a decline
in organized thoughts, leading to speech impairment and an inability to
do appropriate word sequencing and a compromised ability to understand
written and spoken words, as well as memory loss.13 Stroke
patients experience varied levels of memory loss, but the elderly are
more prone to severe memory loss, or in extreme cases, dementia.
Anosognosia is a cognitive dysfunction that manifests as lack of
adequate insight or understanding about the aftermath of stroke caused
by damage to pathways for proper information transfer with effects on
cognition.14 Aphasia is impairment of the ability to speak, which affects one-third of patients with acute stroke.15
Spontaneous resolution of aphasia may occur, although half of affected
patients still experience the problem long-term after the stroke.
Apraxia of speech is a motor programming disorder resulting in
distortions, substitutions, and omissions.9
Psychiatric Disturbances
Emotional problems often afflict stroke patients. Among
these are mood disorders such as depression, anxiety, emotional
instability, crisis reaction, and poststroke fatigue.16
Poststroke fatigue is associated with an overwhelming feeling and/or
heightened sensation of physical and/or mental strain in patients who
otherwise do not have severe neurologic or functional impairment.17
Many factors contribute to the development of emotional problems in
stroke patients. Physiological causes include disruption of neuronal
pathways in the neuromaturation centers by direct damage from stroke.
Secondary factors that may cause emotional disturbance include
disability, loss of self-care ability, limited communication ability
aggravated by speech and cognition impairments, and lack of social
support.
MANAGEMENT OPTIONS
Various modalities are utilized to manage, contain, and
limit the neurologic complications from stroke. The key issues to
resolve are always related to the best therapy, patient selection, and
implementation of effective monitoring parameters. Both nonpharmacologic
and pharmacologic methods are appropriate.
Nonpharmacologic Treatment
Rehabilitation Techniques: Rehabilitation
interventions should begin soon after the stroke in order to be
effective. A wide variety of therapeutic techniques are available, and
selection should be tailored to the patient’s specific deficits, needs,
and response.18 Movement rehabilitation techniques are
useful, and these are aimed at improvements in neural plasticity and
neuromuscular dysfunction. These techniques are task specific and use
movement patterns to enhance the body as a whole, including treadmills
with body weight support, robotic ortheses for upper and lower limbs,
virtual reality technologies, and functional programmed
electromyostimulation. Rehabilitation treatment for spasticity initially
targets the affected limb, using passive stretching and range of motion
exercises of the limb. Splinting and proper positioning may ameliorate
limb spasticity.19 Behavioral management techniques are used
in incontinence rehabilitation. Among these are prompted voiding and
bladder retraining.11,12
Speech and language therapy (SLT) is the recommended therapy for patients with aphasia.20,21
Intensive SLT over a short duration has been shown to produce better
outcomes than less intensive therapy. Intensive SLT involves training
that uses frequent high-intensity and prolonged exercises. The efficacy
of intense speech rehabilitation is supported by several studies.20,21
New therapies such as constraint-induced therapy, suppressing nonverbal
communication, and transcranial magnetic stimulation (TMS) to cortical
language areas have shown promise.22-24
Incorporation of restorative therapies during later stages
of rehabilitation has been shown to be beneficial. Restorative
therapies include growth factors, cell-based therapies, electromagnetic
stimulation, device-based strategies, and task-oriented and repetitive
training–based interventions. Psychotherapies are utilized for emotional
rehabilitation of stroke patients. These techniques include
motivational interviewing and cognitive behavioral therapies.18
Pharmacologic Treatment
Thrombolytic Therapy to Stop Neurologic Deterioration: Preventing
and improving neurologic sequelae of stroke requires early and rapid
reestablishing of blood flow in affected cerebral blood vessels and
reperfusing of cerebral tissues. Thrombolytic therapy using IV
recombinant tissue plasminogen activator (rtPA) within 4.5 hours of
symptom onset is the preferred and currently recommended therapy of the
American Heart Association and American Stroke Association (AHA/ASA)
guidelines.25,26 The use of rtPA is associated with best
possible outcome if patients receive therapy within 90 minutes, and the
benefit has been shown to persist longer than a year. A major side
effect of treatment with IV rtPA is intracranial bleeding; as such,
several ongoing monitoring procedures should be deployed including
neurologic assessments every 15 minutes while administering the
medication and every 30 minutes thereafter. Intracranial hemorrhage
should be highly suspected if patients develop severe headache, acute
hypertension, worsening of neurologic deficit, nausea, vomiting, or
decreased level of consciousness. The administration of rtPA should be
stopped as soon as there are any symptoms of intracranial hemorrhage,
and a thorough neurologic examination should be conducted. Additionally,
AHA/ASA guidelines support rtPA therapy before the results of
coagulation studies are available, except if the patient has a bleeding
disorder or suspected thrombocytopenia. Seizure patients can receive
rtPA therapy after imaging results indicate that the neurologic deficits
are due to cerebral ischemia alone.27,28 Getting informed
consent from patients or their families is not mandatory before
initiating rtPA therapy, but families should be advised about the risks,
benefits, and alternatives to treating with rtPA.25
Other thrombolytic agents have not shown success similar
to that of rtPA. Streptokinase causes higher rates of bleeding, while
reteplase, urokinase, and anistreplase have excessive bleeding rates in
patients in clinical trials.29-31 Although tenecteplase and
desmoteplase have shown favorable results in a few studies, they are not
recommended for first-line therapy.10,26,32 Secondary
preventive prophylaxis with antiplatelet medications such as aspirin can
be started 24 hours after the use of rtPA and completion of cerebral
imaging evaluation for hemorrhage. The use of anticoagulation agents is
not recommended in acute ischemic stroke in the hope of preventing
recurrence, limiting neurologic worsening, or improving outcomes.
Antiplatelet agents may be used long-term after the stroke
event to prevent recurrence. Antiplatelets may have beneficial effects
in secondary prevention; these include ticlopidine 250 mg/day orally;
clopidogrel 300 mg oral loading dose followed by 75 mg/day orally; and
dipyridamole/aspirin combination (200 mg dipyridamole/25 mg aspirin) 1
capsule/day orally.25
Treating Neuromuscular Dysfunction
Neuromuscular dysfunction such as apraxia can be managed
with dopaminergic antispasticity agents and anticholinesterase
inhibitors. For spasticity, the initial treatment is nonpharmacologic,
but adjunctive therapy with baclofen, tizanidine, dantrolene, or
clonazepam is often necessary. Patients who have not achieved adequate
response may require baclofen pump implants and botulinum toxin type A
injections.8 Pain syndromes management strategies include use
of analgesics and nonsteroidal anti-inflammatory drugs (NSAIDs) if
there are no contraindications. Aggressive therapies include use of
corticosteroid injections mixed with lidocaine in a local injection into
the subacromial bursa or other areas affected. Consideration should be
given to administering a lower dose of the corticosteroid in patients
with diabetes to prevent hyperglycemia.10,33,34 CPSP is
managed with antidepressants including amitriptyline and nortriptyline
and antiepileptics such as gabapentin, pregabalin, carbamazepine, and
opioids.35-38 Management for post-stroke urinary and bowel
incontinence includes the use of medications such as oxybutynin that
work on stopping the uninhibited contractions of the detrusor muscle and
delay voiding.11
Therapy Options for Cognitive Problems
There are limited treatment options for anosognosia, which
include the use of antidepressants like nortriptyline and fluoxetine
for preventing patient decline and achieving better out-comes.39
The recovery of language ability is dependent on reestablishing
reperfusion to the ischemic penumbra region of the stroke, resolving
edema, and improving metabolism in the affected cerebral tissues. The
mech-anisms to restore the brain processes to improve aphasia can occur
spontaneously or be aided by achieving successful reperfusion. In
addition, drugs that potentiate language function through reconnecting
neurotransmitter activity, such as donepezil, have been reported to be
useful.40
Treating Psychiatric Disturbances
Selective serotonin reuptake inhibitors (SSRIs) remain
first-line therapy for the treatment of poststroke depression. A
meta-analysis of several studies showed that antidepressants can
effectively improve depression symptoms and help stroke patients to
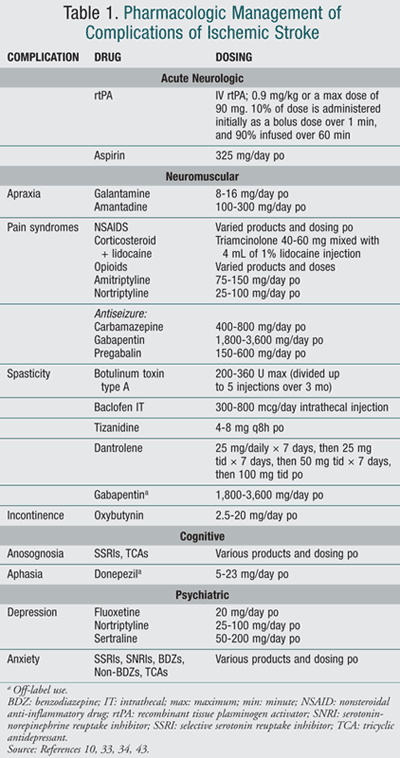
achieve better rehabilitation outcomes and functional recovery.41,42 TABLE 1 summarizes pharmacologic treatment options.43

Potential Medications To Be Avoided in the Elderly
Based on the American Geriatrics Society 2012 Beers
Criteria, providers should exercise caution in the selection and use of
medications for treating patients ≥65 years.44 Accordingly,
benzodiazepines such as clonazepam may be inappropriate for use in the
elderly because of the high incidence of adverse outcomes. Similarly,
NSAIDs should be used on a short-term basis only, and tricyclic
antidepressants should be used with caution and monitoring.
FUTURE TRENDS
The future management of poststroke sequelae may focus on
cerebral vascular protection to decrease the stroke infarct size and
central nervous system stimulation in an effort to promote
neuroprotection and improve stroke outcomes. Agents with the potential
to decrease the size of the stroke infarct include statins, angiotensin
receptor blockers (e.g., candesartan, atorvastatin), minocycline,
cholinesterase inhibitors, and methylphenidate. Finally, amantadine has
been shown to improve speech rehabilitation.45,46
CONCLUSION
Stroke remains a leading cause of morbidity, mortality,
and disability. Impacting the outcomes of stroke requires urgent removal
of thrombotic blockages in cerebral arteries and reestablishing blood
flow and reperfusion to limit the damage to cerebral tissues. Ischemic
stroke is accompanied by neurologic sequelae that may require both
pharmacologic management and intensive rehabilitation techniques
tailored to the patient’s needs and response.
REFERENCES
1. Adams HP Jr, Bendixen BH, Kappelle LJ, et al.
Classification of subtype of acute ischemic stroke: definitions for use
in a multicenter clinical trial, TOAST, Trial of Org 10172 in Acute
Stroke Treatment. Stroke. 1993;24:35-41.
2. Yuan J. Neuroprotective strategies targeting apoptotic and necrotic cell death for stroke. Apoptosis. 2009;14:469-477.
3. Baron JC. Perfusion thresholds in human cerebral ischemia: historical perspective and therapeutic implications. Cerebrovasc Dis. 2001;11(suppl 8):2-8.
4. Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229-317.
5. CDC. Prevalence and most common causes of disability among adults–United States, 2005. MMWR Morb Mortal Wkly Rep. 2009;58:421-426.
6. Kelly-Hayes M, Beiser A, Kase CS, et al. The influence
of gender and age on disability following ischemic stroke: the
Framingham study. J Stroke Cerebrovasc Dis. 2003;12:119-126.
7. Weimar C, Mieck T, Buchthal J, et al; German Stroke
Study Collaboration. Neurologic worsening during the acute phase of
ischemic stroke. Arch Neurol. 2005;62:393-397.
8. Young JA, Tolentino M. Stroke evaluation and treatment. Topics Stroke Rehabil. 2009;16:389-410.
9. Donkervoort M, Dekker J, Deelman B. The course of
apraxia and activities of daily living functioning in left hemisphere
stroke patients
treated in rehabilitation centers and nursing homes. Clin Rehabil. 2006;20:1085-1093.
10. Chae J, Wolf-Johnson T, Govil H. Subacromion
corticosteroid injection for post-stroke shoulder pain: a retrospective
chart review. Arch Phys Med Rehabil. 2007;88:1690-1693.
11. Jordan LA, Mackay E, Coughlan K, et al. Continence management in acute stroke: a survey of current practices in Australia. J Adv Nurs. 2011;67:94-104.
12. Patel M, Coshall C, Rudd A, Wolfe C. Natural history and effects on 2 year outcomes of urinary incontinence after stroke. Stroke. 2001;32:122-127.
13. Vukovic M, Vussanovic J, Vucovick I. Comparison of the
recovery pattern of language processing and cognitive functions in
patients with aphasia following a stroke. J Comm Disord. 2008;41:531-552.
14. Orfer MD, Roberison RG, Prigatamo GP, et al.
Anosognosia for hemiplegia after stroke is a multifaceted phenomenon: a
systematic review of the literature. Brain. 2007;130:3075-3090.
15. Engelter ST, Gostynski M, Papa S, et al. Epidemiology
of aphasia attributable to first ischemic stroke: incidence, severity,
fluency, etiology, and thrombolysis. Stroke. 2006;37:1379-1384.
16. Annoni J, Staub F, Bruggimann L, et al. Emotional disturbances after stroke. Clin Exp Hypertens. 2006;28:243-249.
17. Staub F, Bogousslavsky J. Fatigue after stroke: a major but neglected issue. Cerebrovasc Dis. 2001;12:75-81.
18. Cramer SC. Repairing the human brain after stroke. II. Restorative therapies. Ann Neurol. 2008;63:549-560.
19. Cicerone K, Dahlberg C, Malec J, et al. Evidence-based
cognitive rehabilitation: updated review of the literature from 1998
through 2002. Arch Phys Med Rehabil. 2005;86:1681-1692.
20. Harnish SM, Neils-Strunjas J, Lamy M, Eliassen JC. Use
of fMRI in the study of chronic aphasia recovery after therapy: a case
study. Top Stroke Rehabil. 2008;15:468-483.
21. Pulvermüller F, Berthier ML. Aphasia therapy on a neuroscience basis. Aphasiology. 2008;22:563-599.
22. Meinzer M, Djundja D, Barthel G, et al. Long-term
stability of improved language functions in chronic aphasia after
constraint-induced aphasia therapy. Stroke. 2005;36:1462-1466.
23. Naeser MA, Martin PI, Nicholas M, et al. Improved
picture naming in chronic aphasia after TMS to part of right Broca’s
area: an open-protocol study. Brain Lang. 2005;93:95-105.
24. Maeda F, Pascual-Leone A. Transcranial magnetic stimulation studying motor neurophysiology of psychiatric disorder. Psychopharmacology. 2003;168:359-376.
25. Adams HP Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke. Stroke. 2007;38:1655-1711.
26. Lesbeskind DS. Reversing stroke in the 2010s. Lessons from DIAS2. Stroke. 2009;40:3156-3158.
27. Sylaja PN, Dzialowski I, Krol A, et al; Calgary Stroke
Program. Role of CT angiography in thrombolysis decision-making for
patients with presumed seizure at stroke onset. Stroke. 2006;37:915-917.
28. Selim M, Kumar S, Fink J, et al. Seizure at stroke onset: should it be an absolute contraindication to thrombolysis? Cerebrovasc Dis. 2002;14:54-57.
29. Donnan GA, Hommel M, Davis SM, McNeil JJ; Steering
Committees of the Australian Streptokinase (ASK) and MAST-E trials.
Streptokinase in acute ischaemic stroke. Lancet. 1995;346:56.
30. Hommel M, Boissel JP, Cornu C, et al; MAST Study
Group. Termination of trial of streptokinase in severe acute ischaemic
stroke. Lancet. 1995;345:57.
31. The Multicenter Acute Stroke Trial–Europe Study Group. Thrombolytic therapy with streptokinase in acute ischemic stroke. N Engl J Med. 1996;335:145-150.
32. Haley EC Jr, Lyden PD, Johnston KC, Hemmen TM; TNK in
Stroke Investigators. A pilot dose-escalation safety study of
tenecteplase in acute ischemic stroke. Stroke. 2005;36:607-612.
33. Chae J, Jedlicka L. Subacromial corticosteroid
injection for poststroke shoulder pain: an exploratory prospective case
series. Arch Phys Med Rehabil. 2009;90:501-506.
34. Lakse E, Gunduz B, Erhan B, Celik EC. The effect of
local injections in hemiplegic shoulder pain: a prospective, randomized,
controlled study. Am J Phys Med Rehabil. 2009;88:805-814.
35. Gordon A. Best practice guidelines for treatment of central pain after stroke. In: Henry JL, Panju A, Yashpal K, eds. Central Neuropathic Pain: Focus on Poststroke Pain. Seattle, WA: IASP Press; 2007.
36. Lampl C, Yazdi K, Röper C. Amitriptyline in the
prophylaxis of central poststroke pain. Preliminary results of 39
patients in a placebo-controlled, long-term study. Stroke. 2002;33:3030-3032.
37. Rowbotham MC, Twilling L, Davies PS, et al. Oral opioid therapy for chronic peripheral and central neuropathic pain. N Engl J Med. 2003;348:1223-1232.
38. Frese A, Husstedt IW, Ringelstein EB, Evers S. Pharmacologic treatment of central post-stroke pain. Clin J Pain. 2006;22:252-260.
39. Barrett AM, Crucian GP, Schwartz RL, Heilman KM.
Adverse effect of dopamine agonist therapy in a patient with
motor-intentional neglect. Arch Phys Med Rehabil. 1999;80:600-603.
40. Hillis AE. Rehabilitation of unilateral spatial neglect: new insights from magnetic resonance perfusion imaging. Arch Phys Med Rehabil. 2006;87(suppl 2):S43-S49.
41. Rayner L, Price A, Evans A, et al. Antidepressants for depression in physically ill people. Cochrane Database Syst Rev. 2010;(3):CD007503.
42. Turner-Stokes L, Hassan N. Depression after stroke: a
review of the evidence base to inform the development of an integrated
care pathway. Part 2: Treatment alternatives. Clin Rehabil. 2002;16:248-260.
43. Physicians’ Desk Reference. Montvale, NJ: PDR Network, LLC; 2012. www.pdr.net/drugpages/concisemonograph.aspx. Accessed December 13, 2012.
44. American Geriatrics Society 2012 Beers Criteria Update
Expert Panel. American Geriatrics Society updated Beers Criteria for
potentially inappropriate medication use in the older adults. Am Geriatr Soc. 2012;60:616-631.
45. Guan W, Kozak A, Fagan S. Drug repurposing for vascular protection after acute ischemic stroke. Acta Neurochir Supp. 2011;111:295-298.
46. Barrett A, Levy C, Gonzales Rothi L. Pharmaceuticals for poststroke and brain injury rehabilitation. Am J Phys Med Rehabil. 2007;86:603-604.
To comment on this article, contact [email protected].